Research with Human Participants
The University requires that all research conducted by its members conforms to the highest ethical standards. These standards are defined by the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. As Per TCPS 2 (2022) research involving the following requires ethics review and approval by a Research Ethics Board (REB) before the research commences: living human participants; human biological materials, as well as human embryos, fetuses, fetal tissue, reproductive materials and stem cells. This applies to materials derived from living and deceased individuals; secondary use of data, health information, or biological materials.
Click on the links below for forms and other information:
- Ethics - Research with Humans RCH-020-101
- Research and Scholarly Integrity Policy
- Interagency Advisory Panel on Research Ethics (PRE) - Ethics Tutorial
- Course Based Research Unit Reviews (Undergraduate/ Graduate)
- Guidelines for Course-Based Research Projects (Doc)
Tri-Council Policy Statement (TCPS) Course on Research Ethics (CORE)
The Panel on Research Ethics (PRE) offers a tutorial program called Course on Research Ethics (CORE). All researchers that will conduct research with human participants must complete the online course to increase their familiarity with the TCPS 2.
REB Information and Deadlines
Please send all correspondence to research.ethics@uregina.ca
- Ethics Policy and Education Coordinator: (Compliance Specialist - Human Ethics )
- Download the Terms of Reference (Word Doc)
- Download the list of REB members (PDF)
- Download REB Full Board Meetings and Deadline for Submissions Schedule (PDF)
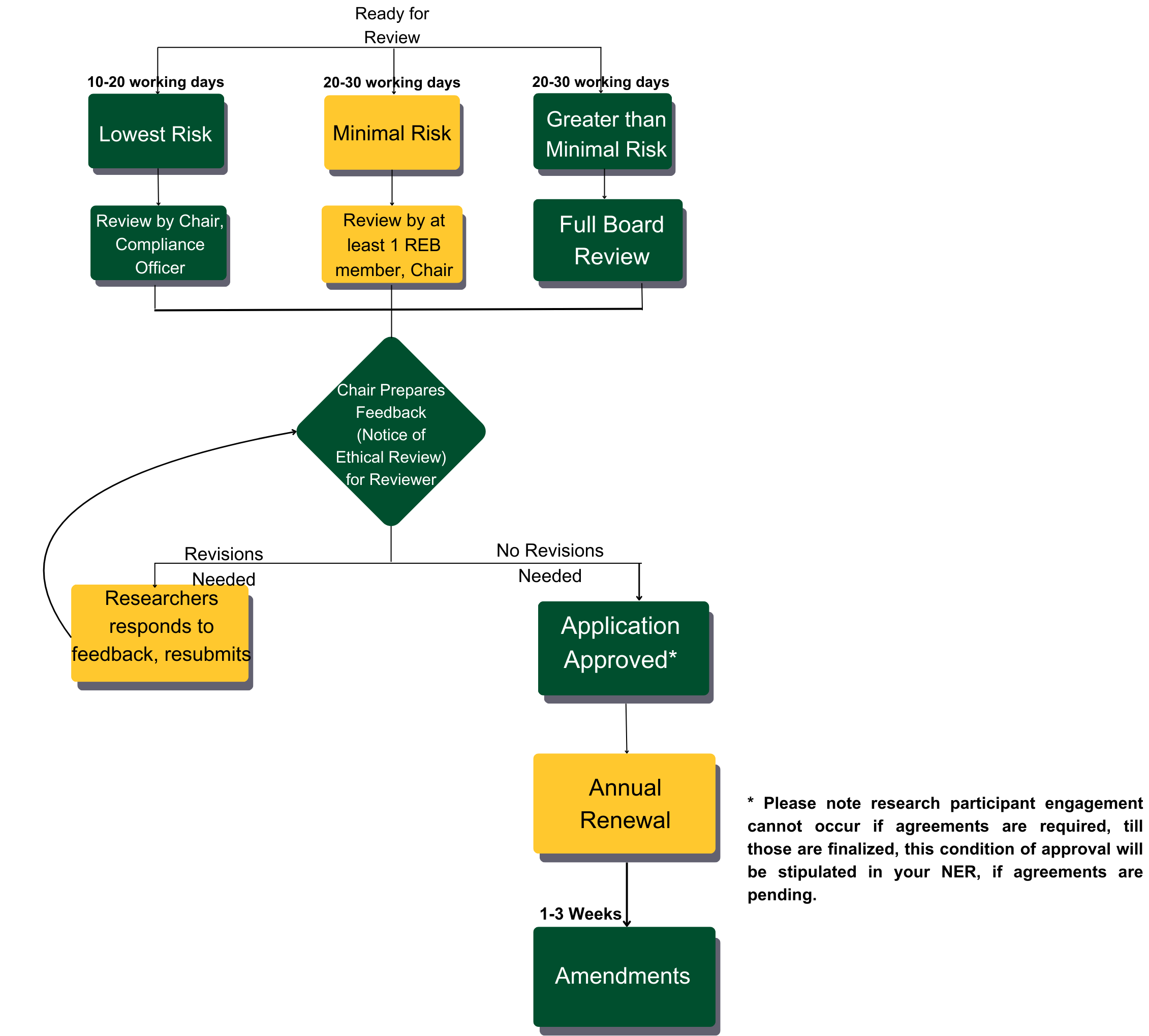
- Research Ethics Board Flowchart
- Research Ethics Board Review - PowerPoint
- Research Ethics Board Review - Recording
For international research projects, if you require the University of Regina’s Federal Worldwide Assurance (FWA) Registration Number please contact research.ethics@uregina.ca