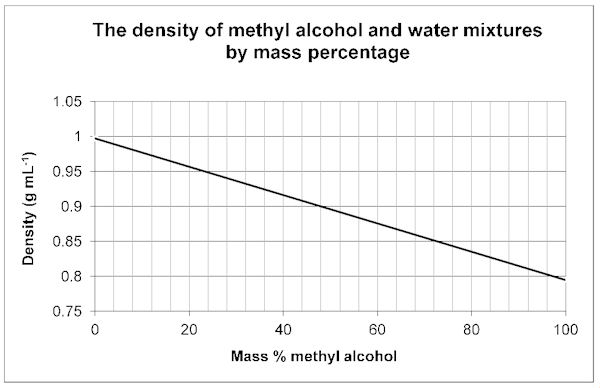Observed Atmospheric Pressure:
Jan 14th : 717.4 mmHg
REMEMBER REPORT QUESTIONS ARE FOUND ON ASSIGNMENTS LINK
Identification of Unknown:
The chemical in your unknown may be any of the following. Identify the chemical by the boiling point. (Note that water in your sample will affect the boiling point.)
Substance Boiling Point ∘C
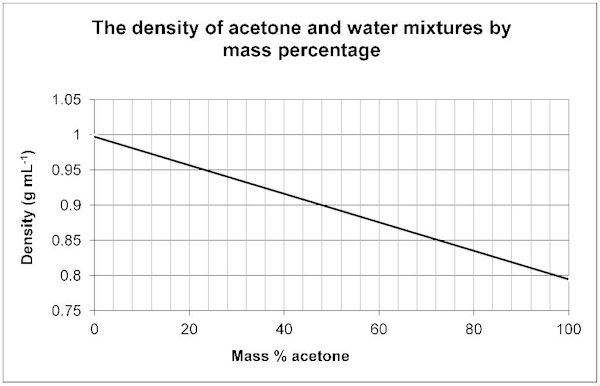
Acetone 56
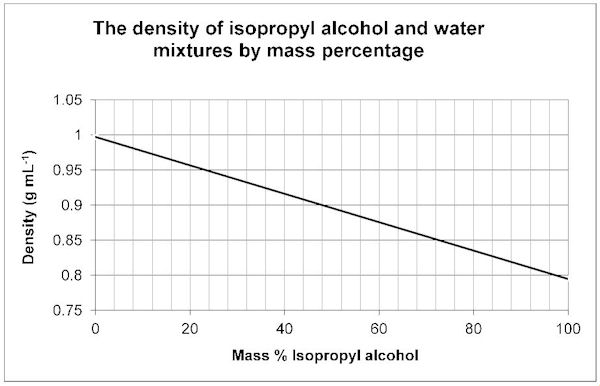
Isopropyl alcohol 83
Methyl alcohol 65
Water 100
Using the Density to determine the Mass % of your Unknown/Water Mixture
Your sample contains an unknown liquid, some salt, and water. The salt content of these samples is 0.009 g/mL. Subtract this from your density that you obtained on your computer graph to determine the density of the water and unknown liquid.
Example: Density from graph is 0.959 g/mL - 0.009 g/mL = 0.950 g/mL
If this was your sample you would use the density of 0.950 g/mL in the charts below. Do your calculation with your own values and then use the chart the corresponds with your unknown. For example, if your boiling point indicated you had Acetone, and your density (without salt) was 0.950 g/mL .... the charts below indicate a Mass % Acetone of 24%.
Use the following graphs to determine the mass % of your Unknown/Water Mixture.